Les sols sont le support de la vie terrestre et le substrat de la végétation. Ce sont des écosystèmes complexes et fragiles qui contribuent à la qualité de notre environnement. Leur étude est, par essence, pluridisciplinaire et se situe au carrefour de la géologie, de la physique, de la chimie, de la biologie, de l'agriculture et de la climatologie. Leurs caractéristiques physicochimiques et biologiques conditionnent la nature de la végétation, la qualité et le rendement des cultures. Les pratiques de l'agriculture intensive et industrielle les appauvrissent considérablement dans nombre de régions du globe, y compris dans notre pays et il convient d'en prendre conscience et de tenter d'y remédier. Les sols contribuent aussi au stockage et au piégeage du gaz carbonique, au travers de la minéralisation de la matière organique, et sont donc un puits de carbone, mais ils peuvent aussi, dans certaines conditions, en libérer et devenir une source supplémentaire de ce gaz à effet de serre. Leur gestion est donc un facteur important à maitriser dans les efforts pour atténuer le changement climatique .
L'objectif de cette séance, commune avec l'Académie d'agriculture de France est de faire un point des connaissances sur quelques aspects de la science des sols et sur les enjeux qui s'y rattachent.
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account

| Tags |
|---|
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
Phosphorus (P), a macronutrient, plays key roles in plant growth, development, and yield. Phosphate (Pi) transporters (PHTs) and PHOSPHATE1 (PHO1) are central to Pi acquisition and distribution. Potentially, PHO1 is also involved in signal transduction under low P. The current study was designed to identify and functionally characterize the PHO1 gene family in chickpea (CaPHO1s). Five CaPHO1 genes were identified through a comprehensive genome-wide search. Phylogenetically, CaPHO1s formed two clades, and protein sequence analyses confirmed the presence of conserved domains. CaPHO1s are expressed in different plant organs including root nodules and are induced by Pi-limiting conditions. Functional complementation of atpho1 mutant with three CaPHO1 members, CaPHO1, CaPHO1;like, and CaPHO1;H1, independently demonstrated their role in root to shoot Pi transport, and their redundant functions. To further validate this, we raised independent RNA-interference (RNAi) lines of CaPHO1, CaPHO1;like, and CaPHO1;H1 along with triple mutant line in chickpea. While single gene RNAi lines behaved just like WT, triple knock-down RNAi lines (capho1/like/h1) showed reduced shoot growth and shoot Pi content. Lastly, we showed that CaPHO1s are involved in root nodule development and Pi content. Our findings suggest that CaPHO1 members function redundantly in root to shoot Pi export and root nodule development in chickpea.
From
link
S. rochei S32 significantly improved the growth of wheat and tomato. The shoot length (24.7%) and root length (25.3%) of wheat (400-fold dilution of cell-free fermentation filtrate) were increased, and the root length of tomato (200-fold dilution) was prolonged (40.9%), and the field yield was also increased. S. rochei S32 showed antagonistic activity against multiple pathogenic fungi, especially Macropoma kawatsukai. The bacterial genome contains an 8,041,158-bp chromosome and two plasmids. A total of 7486 annotated genes were classified into 31 Gene Ontology functional categories. Genomic analysis revealed the potential for the production of indole-3-acetic acid, fungal cell wall hydrolases, antibiotics (e.g., candicidin, streptothricin, borrellin, albaflavenone), and siderophores. Thirty-nine phytohormones and 2205 secondary metabolites were detected, including indole-3-acetic acid, phytosphingosine, acivicin, and corynebactin. Normal bacterial growth occurred on a nitrogen-free medium.
Jean-Michel Ané's insight:
The evidence supporting nitrogen fixation in this bacterium is quite weak...
From
www
Nodulation is the most efficient nitrate assimilation system in the ecosystem, while excessive fertilization has an increased nitrate inhibition effect; deciphering the nitrate signal transduction mechanism in the process is of the utmost importance. In this study, genome-wide analyses of the GmCEP genes were applied to identify nodulation-related CEP genes; 22 GmCEP family members were identified, while GmCEP6 was mainly expressed in nodules and significantly responded to nitrate treatment and rhizobium infection, especially in later stages. Overexpression and CRISPR-Cas9 were used to validate its role in nodulation. We found that GmCEP6 overexpression significantly increased the nodule number, while GmCEP6 knock-out significantly decreased the nodule number, which suggests that GmCEP6 functions as a positive regulator in soybean nodulation. qRT-PCR showed that alterations in the expression of GmCEP6 affected the expression of marker genes in the Nod factor signaling pathway. Lastly, the function of GmCEP6 in nitrate inhibition of nodulation was analyzed; nodule numbers in the GmCEP6-overexpressed roots significantly increased under nitrogen treatments, which suggests that GmCEP6 functions in the resistance to nitrate inhibition. The study helps us understand that GmCEP6 promotes nodulation and participates in the regulation of nitrate inhibition of nodulation, which is of great significance for high efficiency utilization of nitrogen in soybeans.
From
link
Nitrogen (N) is a crucial nutrient for the growth and activity of rhizosphere microorganisms, particularly during drought conditions. Plant root-secreted mucilage contains N that could potentially nourish rhizosphere microbial communities. However, there remains a significant gap in understanding mucilage N content, its source, and its utilization by microorganisms under drought stress. In this study, we investigated the impact of four maize varieties (DH02 and DH04 from Kenya, and Kentos and Keops from Germany) on the secretion rates of mucilage from aerial roots and explored the origin of mucilage N supporting microbial life in the rhizosphere. We found that DH02 exhibited a 96% higher mucilage secretion rate compared to Kentos, while Keops showed 114% and 89% higher secretion rates compared to Kentos and DH04, respectively. On average, the four maize varieties released 4 μg N per root tip per day, representing 2% of total mucilage secretion. Notably, the natural abundance of 15N isotopes increased (higher δ15N signature) with mucilage N release. This indicates a potential dilution of the isotopic signal from biological fixation of atmospheric N by mucilage-inhabiting bacteria as mucilage secretion rates increase. We proposed a model linking mucilage secretion to a mixture of isotopic signatures and estimated that biological N fixation may contribute to 45 - 75% of mucilage N per root tip. The N content of mucilage from a single maize root tip can support a bacterial population ranging from 107 to 1010 cells per day. In conclusion, mucilage serves as a significant N-rich resource for microbial communities in the rhizosphere during drought conditions.
Here, we used in vitro and pot cultivation systems of AM fungi to investigate whether certain keystone bacteria were able to shape the microbial communities growing in the hyphosphere and potentially improved the fitness of the AM fungal host. Based on various AM fungi, soil leachates, and synthetic microbial communities, we found that under organic phosphorus (P) conditions, AM fungi could selectively recruit bacteria that enhanced their P nutrition and competed with less P-mobilizing bacteria. Specifically, we observed a privileged interaction between the isolate Streptomyces sp. D1 and AM fungi of the genus Rhizophagus, where (1) the carbon compounds exuded by the fungus were acquired by the bacterium which could mineralize organic P and (2) the in vitro culturable bacterial community residing on the surface of hyphae was in part regulated by Streptomyces sp. D1, primarily by inhibiting the bacteria with weak P-mineralizing ability, thereby enhancing AM fungi to acquire P.
In the relentless pursuit of sustainable agricultural practices, society has pivoted its gaze towards alternatives to synthetic chemical fertilizers, recognizing the significant environmental impact they impose. Among the myriad of alternatives, the use of plant growth-promoting bacteria (PGPB) has emerged as a promising solution, encouraging potential to revolutionize plant nutrition in a manner that is both effective and environmentally sustainable. The interaction between plants and PGPB is a wonder of nature, encompassing a wide array of interactions that extend far beyond simple nutrient provision. These remarkable microorganisms, through their ability to harness unavailable nutrients and synthesize essential phytohormones, exert a profound influence on plant metabolism, enhancing growth and resilience even in challenging conditions.
From
academic
The REQUIRED FOR ARBUSCULAR MYCORRHIZATION1 (RAM1) transcription factor from the GRAS family is well-known by its role as a master regulator of the arbuscular mycorrhizal (AM) symbiosis in dicot and monocot species, being essential in the transcriptional reprograming for the development and functionality of the arbuscules. In tomato, SlGRAS27 is the putative ortholog of RAM1 (here named SlRAM1), but has not yet been characterized. A reduced colonization of the root and an impaired arbuscule formation were observed in the SlRAM1 silenced plants, confirming the functional conservation of the RAM1 ortholog in tomato . However, unexpectedly, SlRAM1 overexpressing (UBIL:SlRAM1) plants also showed a decreased mycorrhizal colonization. Analysis of non-mycorrhizal UBIL:SlRAM1 roots revealed an overall regulation of AM-related genes and a reduction of strigolactone biosynthesis. Moreover, the external application of the strigolactone analogue GR244DO almost completely reversed the negative effects of SlRAM1 overexpression on the frequency of mycorrhization. However, it only partially recovered the pattern of arbuscule distribution observed in control plants. Our results strongly suggest that SlRAM1 has a dual regulatory role during mycorrhization and, apart from its recognized action as a positive regulator of arbuscule development, SlRAM1 is also involved in different mechanisms for the negative regulation of mycorrhization, including the repression of strigolactone biosynthesis.
Although plants are sessile, their ubiquitous distribution, ability to harness energy from the sun, and ability to sense above and belowground signals make them ideal candidates for biosensor development. Synthetic biology has allowed scientists to reimagine biosensors as engineered devices that are focused on accomplishing novel tasks. As such, a new wave of plant-based sensors, phytosensors, are being engineered as multi-component sense-and-report devices that can alert human operators to a variety of hazards. While phytosensors are intrinsically tied to agriculture, a new generation of phytosensors has been envisioned to function in the built environment and even in austere environments, such as space. In this review, we will explore the current state of the art with regard to phytosensor engineering.
•The quantity and activity of nutrient acquisition agents, e.g., roots, rhizobium and mycorrhizal fungi, are important for plant growth. How the invasive plants and the native legume adjust the quantity and activity of these agents in competing for soil resources remains unclear.
Jean-Michel Ané's insight:
That makes sense -
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH or Gap) is a ubiquitous enzyme essential for carbon and energy metabolism in most organisms. Despite its primary role in sugar metabolism, GAPDH is recognized for its involvement in diverse cellular processes, being considered a paradigm among multifunctional/moonlighting proteins. Besides its canonical cytoplasmic location, GAPDH has been detected on cell surfaces or as a secreted protein in prokaryotes, yet little is known about its possible roles in plant symbiotic bacteria. Here we report that Rhizobium etli, a nitrogen-fixing symbiont of common beans, carries a single gap gene responsible for both GAPDH glycolytic and gluconeogenic activities. An active Gap protein is required throughout all stages of the symbiosis between R. etli and its host plant Phaseolus vulgaris. Both glycolytic and gluconeogenic Gap metabolic activities likely contribute to bacterial fitness during early and intermediate stages of the interaction, whereas GAPDH gluconeogenic activity seems critical for nodule invasion and nitrogen fixation. Although the R. etli Gap protein is secreted in a c-di-GMP related manner, no involvement of the R. etli gap gene in c-di-GMP related phenotypes, such as flocculation, biofilm formation or EPS production, was observed. Notably, the R. etli gap gene fully complemented a double gap1/gap2 mutant of Pseudomonas syringae for free life growth, albeit only partially in planta, suggesting potential specific roles for each type of Gap protein. Nevertheless, further research is required to unravel additional functions of the R. etli Gap protein beyond its essential metabolic roles.
Jean-Michel Ané's insight:
Mind the gap :-)
From
cdnsciencepub
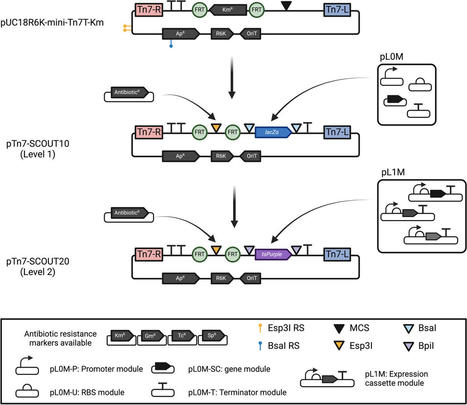
Ammonia availability has a crucial role in agriculture as it ensures healthy plant growth and increased crop yields. Since diazotrophs are the only organisms capable of reducing dinitrogen to ammonia, they have great ecological importance and potential to mitigate the environmental and economic costs of synthetic fertilizer use. Rhizobia are especially valuable being that they can engage in nitrogen-fixing symbiotic relationships with legumes, and they demonstrate great diversity and plasticity in genomic and phenotypic traits. However, few rhizobial species have sufficient genetic tractability for synthetic biology applications. This study established a basic genetic toolbox with antibiotic resistance markers, multi-host shuttle plasmids and a streamlined protocol for biparental conjugation with Mesorhizobium and Bradyrhizobium species. We identified two repABC origins of replication from Sinorhizobium meliloti (pSymB) and Rhizobium etli (p42d) that were stable across all three strains of interest. Furthermore, the NZP2235 genome was sequenced and phylogenetic analysis determined its reclassification to Mesorhizobium huakuii. These tools will enable the use of plasmid-based strategies for more advanced genetic engineering projects and ultimately contribute towards the development of more sustainable agriculture practices by means of novel nitrogen-fixing organelles, elite bioinoculants, or symbiotic association with nonlegumes.
Jean-Michel Ané's insight:
Super useful toolkit. Thank you @jordynsmeaney, @George_diCenzo, @KarasLab and colleagues for this great resource!
Although the molecular mechanism underlying IT development remains obscure, several host players have been characterized, including VAPYRIN (VPY). The vpy mutant shows delayed initiation of ITs, often resulting in oversized MCs (Murray et al., 2011; Liu et al., 2019). VPY is also required for arbuscule development in arbuscular mycorrhiza (AM) symbiosis (Feddermann et al., 2010; Pumplin et al., 2010; Murray et al., 2011; Zhang et al., 2015; Bapaume et al., 2019; Chen et al., 2021). VPY encodes a plant-specific protein with a major sperm protein (MSP) domain and several ankyrin repeats, both of which are protein–protein interaction domains (Tarr & Scott, 2005; Li et al., 2006). In the context of nodulation, VPY interacts with LIN, a protein also essential for IT development, via its ankyrin repeats region, and is positively regulated by LIN, likely by stabilizing VPY protein by so far unknown mechanisms, in both M. truncatula and L. japonicus (Kuppusamy et al., 2004; Kiss et al., 2009; Yano et al., 2009; Liu et al., 2019; Liu et al., 2021). VPY and LIN form an ‘infectosome’ protein complex with RHIZOBIUM-DIRECTED POLAR GROWTH (RPG) and an exocyst subunit EXO70H4, regulating IT development, likely via polarized exocytosis (Arrighi et al., 2008; Liu et al., 2019; Roy et al., 2020; Wang et al., 2022; Jhu & Oldroyd, 2023; Lace et al., 2023; Li et al., 2023). The core infectosome components, VPY, LIN and RPG accumulate at the extending IT tip and form one or a few cytoplasmic puncta near the nucleus (Liu et al., 2019; Lace et al., 2023; Li et al., 2023). The functional significance and mechanism underlying the interaction of these infectosome components remain to be elucidated. |
• Increase of nitrogen use efficiency (NUE) through management and genetic manipulation is an urgent need.
From
link
Root-associated microbiota profoundly affect crop health and productivity. Plants can selectively recruit beneficial microbes from the soil and actively balance microbe-triggered plant-growth promotion and stress tolerance enhancement. The cost associated with this is the root-mediated support of a certain number of specific microbes under nutrient limitation. Thus, it is important to consider the dynamic changes in microbial quantity when it comes to nutrient condition-induced root microbiome reassembly. Quantitative microbiome profiling (QMP) has recently emerged as a means to estimate the specific microbial load variation of a root microbiome (instead of the traditional approach quantifying relative microbial abundances) and data from the QMP approach can be more closely correlated with plant development and/or function. However, due to a lack of detailed-QMP data, how soil nutrient conditions affect quantitative changes in microbial assembly of the root-associated microbiome remains poorly understood. A recent study quantified the dynamics of the soybean root microbiome, under unbalanced fertilization, using QMP and provided data on the use of specific synthetic communities (SynComs) for sustaining crop productivity. In this editorial, we explore potential opportunities for utilizing QMP to decode the microbiome for sustainable agriculture.
Jean-Michel Ané's insight:
Very good review
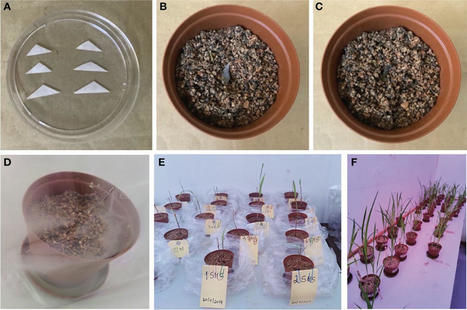
Taxonomic identification of arbuscular mycorrhizal (AM) fungal spores extracted directly from the field is sometimes difficult because spores are often degraded or parasitized by other organisms. Single-spore inoculation of a suitable host plant allows for establishing monosporic cultures of AM fungi. This study aimed to propagate AM fungal spores isolated from maize soil using single spores for morphological characterization. First, trap cultures were established to trigger the sporulation of AM fungal species. Second, trap cultures were established with individual morphotypes by picking up only one spore under a dissecting microscope and transferring it to a small triangle of sterilized filter paper, which was then carefully inoculated below a root from germinated sorghum seeds in each pot and covered with a sterile substrate. All pots were placed in sunbags and maintained in a plant growth room for 120 days. Spores obtained from single spore trap cultures from each treatment, maize after oats (MO), maize after maize (MM), maize after peas (MP), and maize after soybean (MS), were extracted using the sieving method. Healthy spores were selected for morphological analysis. Direct PCR was conducted by crushing spores in RNAlater and applying three sets of primer pairs: ITS1 × ITS4, NS31 × AML2, and SSUmcf and LSUmBr. Nucleotide sequences obtained from Sanger sequencing were aligned on MEGA X. The phylogenetic tree showed that the closest neighbors of the propagated AM fungal species belonged to the genera Claroideoglomus, Funneliformis, Gigaspora, Paraglomus, and Rhizophagus. The morphological characteristics were compared to the descriptive features of described species posted on the INVAM website, and they included Acaulospora cavernata, Diversispora spurca, Funneliformis geosporus, Funneliformis mosseae, Gigaspora clarus, Gigaspora margarita, Glomus macrosporum, Paraglomus occultum, and Rhizophagus intraradices. These findings can provide a great contribution to crop productivity and sustainable management of the agricultural ecosystem. Also, the isolate analyzed could be grouped into efficient promoters of growth and mycorrhization of maize independent of their geographical location.
The rhizosphere system of plants hosts a diverse consortium of bacteria that confer beneficial effects on plant, such as plant growth-promoting rhizobacteria (PGPR), biocontrol agents with disease-suppression activities, and symbiotic nitrogen fixing bacteria with the formation of root nodule. Efficient colonization in planta is of fundamental importance for promoting of these beneficial activities. However, the process of root colonization is complex, consisting of multiple stages, including chemotaxis, adhesion, aggregation, and biofilm formation. The secondary messenger, c-di-GMP (cyclic bis-(3′-5′) dimeric guanosine monophosphate), plays a key regulatory role in a variety of physiological processes. This paper reviews recent progress on the actions of c-di-GMP in plant beneficial bacteria, with a specific focus on its role in chemotaxis, biofilm formation, and nodulation.
Arbuscular mycorrhizal fungi (AMF) are beneficial root symbionts that form mutual partnerships with approximately 90% of plants. They provide water, nutrients, and protection from stresses while receiving photosynthetic products from the host plants. These fungi are essential components of the soil ecosystem, and their absence or decline can negatively impact ecosystem efficiency. In chickpea cultivation, the interaction between AMF and rhizobium is vital for soil processes and plant productivity. Alongside other beneficial microorganisms in the rhizosphere, they enhance the acquisition of essential nutrients like nitrogen (N) and phosphorus (P), promoting chickpea growth and development. These interactions are particularly crucial in low-input, eco-friendly agricultural systems that rely on biological processes to sustain soil quality and productivity without heavy use of agrochemicals. The combination of root nodules' N-fixation and AMF synergism also improves plant P nutrition and stimulates the proliferation of phosphate-solubilizing fungi. However, genetic diversity among native strains and their genes/enzymes can influence the interactions between AMF and rhizobium. To achieve sustainable chickpea production, it is crucial to gain a deeper understanding of these interactions, allowing optimized combinations of microorganisms to be used as effective soil inoculants for promoting plant growth and fitness. This review aims to provide insights into the mechanistic interactions of AMF and rhizobium, their impact on rhizosphere soil health, and the role of environmental factors in regulating chickpea productivity and sustainability.
From
www
Symbiotic nitrogen fixation in legume nodules requires substantial energy investment from host plants, and soybean (Glycine max (L.) supernodulation mutants show stunting and yield penalties due to overconsumption of carbon sources. We obtained soybean mutants differing in their nodulation ability, among which rhizobially induced cle1a/2a (ric1a/2a) has a moderate increase in nodule number, balanced carbon allocation, and enhanced carbon and nitrogen acquisition. In multi-year and multi-site field trials in China, two ric1a/2a lines had improved grain yield, protein content and sustained oil content, demonstrating that gene editing towards optimal nodulation improves soybean yield and quality.
From
www
The fight against biopiracy -- plundering genetic resources and the traditional knowledge surrounding them -- could soon be based on an international treaty which is being finalised at negotiations that began on Monday.
From
www
Nitrogen (N2) fixation in oligotrophic surface waters is the main source of new nitrogen (N) to the ocean1 and plays a key role in fueling the biological carbon pump2. Oceanic N2 fixation is almost exclusively attributed to cyanobacteria, even though genes encoding nitrogenase, the enzyme fixing N2 into ammonia, are widespread among marine bacteria and archaea3-5. Little is known about these non-cyanobacterial N2-fixers and direct proof that they can fix N in the ocean is missing. Here we report the discovery of a non-cyanobacterial N2-fixing symbiont, Candidatus Tectiglobus diatomicola, which provides its diatom host with fixed-N in return for photosynthetic carbon. The N2-fixing symbiont belongs to the order Rhizobiales and its association with a unicellular diatom expands the known hosts for this order beyond the well-known N2-fixing rhizobia-legume symbioses on land6. Our results show that the rhizobia-diatom symbiosis can contribute as much fixed-N as cyanobacterial N2-fixers in the tropical North Atlantic, and that they may be responsible for N2 fixation in the vast regions of the ocean where cyanobacteria are too rare to account for the measured rates.
Jean-Michel Ané's insight:
Eew... The use of the term "rhizobia" here seems inappropriate to me. To call something "rhizobia" one need to demonstrate that it forms a root nodule symbiosis. Not all Rhizobiales (like Agrobacterium) are rhizobia!
Background
Jean-Michel Ané's insight:
Very useful tool for SynComs developed by @beajorrin @PooleLabOxford and coll.
From
link
A novel bacterial symbiont, strain A19T, was previously isolated from a root-nodule of Aeschynomene indica and assigned to a new lineage in the photosynthetic clade of the genus Bradyrhizobium. Here data are presented for the detailed genomic and taxonomic analyses of novel strain A19T. Emphasis is placed on the analysis of genes of practical or ecological significance (photosynthesis, nitrous oxide reductase and nitrogen fixation genes). Phylogenomic analysis of whole genome sequences as well as 50 single-copy core gene sequences placed A19T in a highly supported lineage distinct from described Bradyrhizobium species with B. oligotrophicum as the closest relative. The digital DNA-DNA hybridization and average nucleotide identity values for A19T in pair-wise comparisons with close relatives were far lower than the respective threshold values of 70% and ~ 96% for definition of species boundaries. The complete genome of A19T consists of a single 8.44 Mbp chromosome and contains a photosynthesis gene cluster, nitrogen-fixation genes and genes encoding a complete denitrifying enzyme system including nitrous oxide reductase implicated in the reduction of N2O, a potent greenhouse gas, to inert dinitrogen. Nodulation and type III secretion system genes, needed for nodulation by most rhizobia, were not detected. Data for multiple phenotypic tests complemented the sequence-based analyses. Strain A19T elicits nitrogen-fixing nodules on stems and roots of A. indica plants but not on soybeans or Macroptilium atropurpureum. Based on the data presented, a new species named Bradyrhizobium ontarionense sp. nov. is proposed with strain A19T (= LMG 32638T = HAMBI 3761T) as the type strain.
A trait is an attribute influencing an organism’s performance within its environment, encompassing morphological, genetic and physiological characteristics measured at the individual or population levels (Salguero-Gómez et al. , 2018; Zhang et al. , 2023a). Understanding the ecology of species using a trait-based approach can contribute to a mechanistic explanation of processes mediated by microbes, including those that affect ecosystem functioning (Romero-Olivares et al. , 2021). This approach holds particular significance for arbuscular mycorrhizal (AM) fungi - Phylum Glomeromycota. As obligate symbionts of plants, where multiple species colonize both roots and soils in a network, predicting the functional outcomes (e.g., host growth, plant community diversity, soil characteristics) of individual AM fungal genotypes and communities within ecosystems remains challenging, despite major developments in molecular methods in the last two decades (Tisserant et al. , 2013; Montoliu-Nerin et al. , 2021). Indeed, establishing connections between AM fungal taxa and/or genotypes (e.g., within species) and their functional roles is a laborious process, which is expected to continue in the foreseeable future (Serghi et al. , 2021; Manley et al. , 2023). This is needed due to the complex links between AM fungi and functional outcomes for both hosts (e.g. , plant growth and fitness, nutrient uptake and stress tolerance) and soil functions/properties (e.g. , carbon storage, aggregate stability, biotic diversity), which appear to be highly context dependent and relatively poorly predicted by taxonomy alone (Munkvold et al. , 2004; Koch et al. , 2017; Yang et al. , 2017; Qiu et al. , 2021). However, this effort is also required because AM fungal traits have not been systematically assessed alongside with hypotheses of adaptation or with specific mechanisms in mind. For example, small-spored AM fungi may be dispersed longer distances by wind than large-spored AM fungi. It is then a reasonable hypothesis that small spore size is an adaptation for wind dispersal. One could empirically observe that small-spored AM fungi are geographically more widespread than large-spored fungi and this potential result could be viewed as evidence in support of this hypothesis. However, this finding would not necessarily prove that such dispersal difference has “functional” or “adaptive” value. Alternatively, producing small spores is a correlated response to producing many spores quickly, which itself could be an adaptive response to the likelihood of unpredictable soil disturbance such as from tillage. In this scenario, the adaptation and/or function is the production of many spores quickly to confer resistance to disturbance and then, after soil disturbance with wind erosion, small spores may also be blown farther (which may or may not improve fitness). Another example, variation in rooting depth among plants in a community may contribute to resource partitioning. But the mechanism (differential resource depletion with depth) still needs to be demonstrated separately from the trait evidence. AM fungi could contribute to equalize resource partitioning if plants with short roots associate with AM fungi that form more extensive extra-radical mycelium and vice-versa. Given these complexities, we consider the development of a robust, universally applicable trait-based framework for predicting key AM fungal functional outcomes a priority. To achieve this objective, first we must identify AM fungal traits that can be measured at morphological, physiological, and genetic levels. Second, considering the important roles of AM fungi in ecosystems, affecting host plants, soil, and the AM fungi themselves, we need to discern/hypothesize how measuring AM fungal traits impacts each of these components. For the host plant, it is crucial to consider nutrition, biomass, fitness, and survival in face of pathogens, heavy metals, salinity, drought, etc. (Delavaux et al. , 2017; Wehneret al. ). Within the soil environment, AM fungal effects on soil structure (Rillig & Mummey, 2006), nutrient cycling, carbon storage, and other members of the soil food-web are paramount (Antunes & Koyama, 2016; Frew et al. , 2021; Horsch et al. , 2023a). Regarding the fungal organism, we should focus on key aspects of their life-history strategies; reproduction and fitness, survival, dispersal, competitive ability, infectivity and abundance both within the host and soil environments (Aguilar-Trigueros et al. , 2019; Chaudharyet al. , 2020; Deveautour et al. , 2020). This requires identifying relevant proxies (sometimes termed “soft traits” in the plant ecophysiology literature) to provide easy-to-measure quantitative metrics for such complex facets of fungal life-history that can be measured across several species. Third, we need to evaluate existing standardized methods and experimental designs, or develop new ones, to measure such relevant (soft) traits, as has been done in plant ecophysiology (Pérez-Harguindeguy et al. , 2013). Measurement standardization and relevant metadata for hypothesis-driven analysis and interpretation is essential if we are to aggregate trait information from different studies into a public database, facilitating their incorporation into earth system models (e.g., (Fry et al. , 2019) and enhancing the predictability of functional processes and/or adaptations associated with AM fungi. Analogous libraries on plant traits (Kattge et al. , 2020) have proved useful to better understand trait variation along global climatic gradients (Butleret al. , 2017). Here, we aim to:
Jean-Michel Ané's insight:
A bit dry but useful
From
academic
Molecular innovations within key metabolisms can have profound impacts on element cycling and ecological distribution. Yet, much of the molecular foundations of early evolved enzymes and metabolisms are unknown. Here, we bring one such mystery to relief by probing the birth and evolution of the G-subunit protein, an integral component of certain members of the nitrogenase family, the only enzymes capable of biological nitrogen fixation. The G-subunit is a Paleoproterozoic-age orphan protein that appears more than 1 billion years after the origin of nitrogenases. We show that the G-subunit arose with novel nitrogenase metal dependence and the ecological expansion of nitrogen-fixing microbes following the transition in environmental metal availabilities and atmospheric oxygenation that began ∼2.5 billion years ago. We identify molecular features that suggest early G-subunit proteins mediated cofactor or protein interactions required for novel metal dependency, priming ancient nitrogenases and their hosts to exploit these newly diversified geochemical environments. We further examined the degree of functional specialization in G-subunit evolution with extant and ancestral homologs using laboratory reconstruction experiments. Our results indicate that permanent recruitment of the orphan protein depended on the prior establishment of conserved molecular features and showcase how contingent evolutionary novelties might shape ecologically important microbial innovations. |